- AdventHealth Research Institute
NKGen Biotech, Inc., a clinical-stage biotechnology company focused on the development and commercialization of innovative autologous and allogeneic natural killer (“NK”) cell therapeutics, today announced that Anita Fletcher, M.D. has been appointed as the National Principal Investigator (“PI”) for its Phase 2a clinical trial of troculeucel, expanded enhanced autologous NK cell therapy, for the treatment of moderate Alzheimer’s disease (NCT06189963). In addition to Dr. Fletcher’s appointment as National PI, NKGen is pleased to announce AdventHealth Research Institute, Neuroscience Research in Orlando will be the first clinical site on the East Coast with the intent of enrolling moderate stage Alzheimer’s Disease (“AD”) patients in the very near term.
Read more about the announcement and the study by clicking the button below.
Recent News

The Next Frontier: Preventing Type 1 Diabetes Before It Starts
Recent research led by AdventHealth’s Translational Research Institute and others reveals that Type 1 diabetes develops gradually and can now be detected early through blood tests, opening the door to...
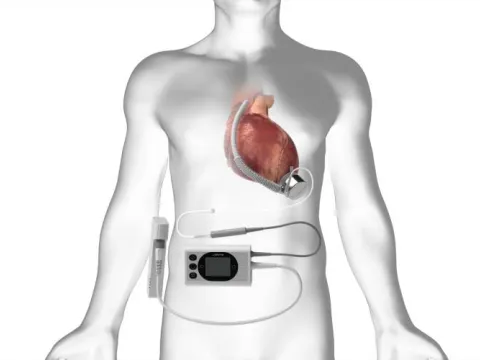
Advancing Heart Care: A New Chapter with the BrioVAD Study
The AdventHealth Research team recently completed Core Training for the INNOVATE 2254936 Vidic trial, bringing this novel device to AdventHealth.

AdventHealth Launches NIH-Funded THRIVE Trial to Address Apathy in Late-Life Depression
AdventHealth Neuroscience Institute is launching THRIVE, a new NIH-funded clinical trial led by geriatric neuropsychologist and neuroscience researcher Lauren Oberlin, PhD.

AdventHealth Leads the Nation in STAR Trial Enrollment
AdventHealth Research Institute (AHRI) is proud to be the highest enrolling site in the United States for the STAR trial.

Gut Microbes, Diet, and Energy: New Clues for Tackling Energy Balance
A controlled dietary study found that a high-fiber, microbiome-enhancing diet altered gut metabolites, reduced energy absorption, and unexpectedly mimicked fasting effects, offering new insights into...

Can Exercise Turn Back the Clock on Your Brain? New Study Says Yes
What if hitting the gym could actually make your brain younger? A new study suggests it can. Our research group found that adults who committed to a year of moderate-to-vigorous aerobic exercise—like...

Dr. Kirk Erickson Featured on Fox 35 to Talk About the Connection Between Physical Activity and Brain Health
AdventHealth Research Institutes own Dr. Kirk Erickson was recently featured on Fox 35 to talk about the connection between physical activity and brain health.

Conform Trial: Major Milestone Achieved
On August 8, 2025, our team successfully completed the very first implant day for the Conform Clinical Trial, enrolling and treating our first three patients with the Conformal CLAAS System for Left...
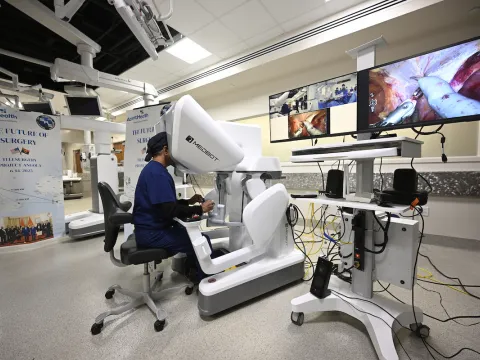
Dr. Vipul Patel Performs the Longest Distance Telesurgery Ever Completed
In our latest Clinician’s View, Dr. Patel shares his team’s telesurgery journey, including how they are ensuring safety, quality and ethical exploration of this innovative approach.

AdventHealth’s Dr. Stephanie Compton Featured in ACS Highlight on Diet and Cancer Treatment
We’re proud to announce that Stephanie Compton, PhD, a valued post‑doctoral researcher at the AdventHealth Research Institute, was recently featured by the American Cancer Society.

Back-to-School with Type 1 Diabetes
Starting school is exciting and for families managing T1D, a little planning can make all the difference.

New Study Enrolling: RISE - Treating Ulcerative Colitis
We are proud to announce the launch of the new RISE study.
