- Kristi Powers
Choose the health content that’s right for you, and get it delivered right in your inbox.

A new way of treating bladder cancer is expected to deliver promising results and the AdventHealth Cancer Institute is the only site in Florida, and the first in the U.S., to open this groundbreaking treatment, known as the INTerpath-005 V940 mRNA vaccine Phase II trial. The treatment became available in April and is aimed at revolutionizing treatment options for those battling this disease.
“This vaccine is precision medicine at its best,” said Dr. Guru Sonpavde, a medical oncologist and the medical director of genitourinary oncology at the AdventHealth Cancer Institute, as well as a member of the trial’s global steering committee. “Unlike most trials where all patients receive one specific drug, some of the patients in this trial will receive a new drug customized to target the specific mutated proteins only found in the cancer cells of that patient.”

This trial introduces a unique and innovative approach to combatting bladder cancer. This patient-customized mRNA vaccine, designed to activate the immune system against the unique proteins present in each individual's tumor, holds immense promise, Sonpavde said. With up to 34 neoantigens – or new mutated proteins encoded in the vaccine – it offers a tailored treatment against bladder cancer.
Participants will receive pembrolizumab (KEYTRUDA) combined with the new customized immunotherapy injection. KEYTRUDA alone has previously improved outcomes following surgical removal of aggressive muscle-invasive urothelial cancer.
“I am confident these two drugs will complement each other and kill cancer, therefore improving the cure rate of bladder cancer after it’s been removed,” said Dr. Sonpavde.
Participants receive the KEYTRUDA infusion every six weeks for one year and once the customized sequencing is complete, they get the V940 injection once every three weeks for nine doses, or for roughly six months.
Dr. Sonpavde sees this trial as a win-win for all participants because the other group that receives only KEYTRUDA, still gets a drug that physicians already know works and improves outcomes, instead of receiving a placebo.
"I’m optimistic the combination drug treatment, the V940 vaccine plus KEYTRUDA, in this trial will be safe and successful and will lead to a Phase III trial,” expressed Dr. Sonpavde. “From there, if benefit is confirmed, I envision this treatment to be more widely available worldwide in the near future, which is very exciting that we may soon be able to cure more patients.”
However, bladder cancer patients don’t have to wait years for access to this treatment. The trial can be accessed now through a physician referral and is available at AdventHealth in Orlando.
More information about becoming a participant in this trial can be discussed with the AdventHealth Clinical Research GU Coordinator at ORL.GU.ClinicalTrials@AdventHealth.com. There are eligibility requirements for this trial that offers, not just treatment, but a promise of a better future.
Recent News

AdventHealth Avista opens food pantry to support community health
AdventHealth Avista has taken a significant step toward addressing food insecurity, a key priority identified in its Community Health Needs Assessment by opening a food pantry on its first floor. This...

AdventHealth Porter Earns COPPER Designation, Strengthening Pediatric Emergency Care
AdventHealth Porter is proud to announce that its Emergency Department has earned the Pediatric Advanced COPPER designation, a significant milestone that reflects a deep commitment to providing safe...

Three AdventHealth hospitals in Florida’s Volusia and Flagler counties earn top Leapfrog honors
Residents of Volusia and Flagler counties now have national confirmation of something many rely on every day: safe, high-quality hospital care close to home.
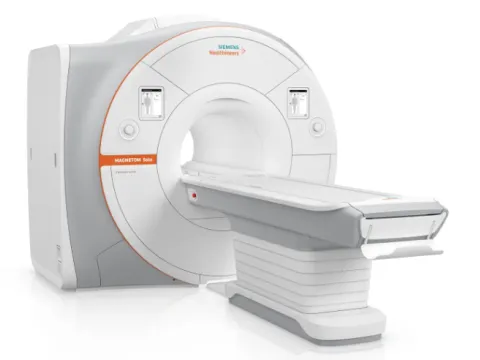
AdventHealth DeLand investing in next-generation MRI technology to enhance patient care
AdventHealth DeLand will soon install a new MRI system designed to deliver faster exams, sharper images and a more comfortable experience for patients in West Volusia.

AdventHealth Wauchula earns 2025 Leapfrog Top Rural Hospital Award for outstanding quality and safety
People in Hardee County can feel confident knowing their local hospital is among the safest in the nation with AdventHealth Wauchula being named a Top Rural Hospital by The Leapfrog Group for its...

Innovative new procedure offers hope for heart transplant candidates at high risk for rejection
Innovation at AdventHealth is driven by one purpose: helping people heal in body, mind and spirit.

NC Supreme Court clears way for first phase of AdventHealth’s new hospital in Weaverville
AdventHealth is grateful for community support as NC Supreme Court decision clears the way for a new hospital.

The hidden cancer one clinician caught – and the process improvements she says matter most
Shana Vongkhankeo, APRN, discovered an unusual thyroid enlargement during a routine physical for a teenage patient, leading to a life-saving cancer diagnosis that highlighted how being fully present...

AdventHealth debuts first-of-its-kind Performer Health Program in Central Florida
AdventHealth is launching the Performer Health Program, a first-of-its-kind initiative in Central Florida focused on addressing the unique health needs of artists and performers.

AdventHealth named among U.S. News & World Report’s fifth annual 2026 Best Hospitals for Maternity Care
This marks the third year in a row AdventHealth Shawnee Mission has appeared on the U.S. News & World Report’s list of Best Hospitals for Maternity Care.

How to stay mindful in body, mind and spirit this holiday season
As holiday demands grow, mindfulness provides a simple tool to stay present and steady, offering support for the mind, body and spirit during a busy season

AdventHealth Waterman strengthens access to expert specialty care with expansion of Mount Dora medical plaza
Second floor buildout adds orthopedics, sports medicine, women’s health and heart care for Lake County.